FCN-005
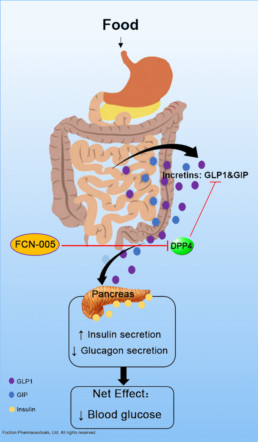
FCN-005 is an orally efficacious, highly potent and selective dipeptidyl peptidase-4 (DPP-4) inhibitor proprietarily developed by Fochon for the treatment of type 2 diabetes.
In pre-clinical studies, FCN-005 exhibited great in vitro and in vivo potency, desirable pharmakinetic/pharmadynamic properties suitable for once daily dosing and high safety margin. In Phase 1 clinical trials, FCN-005 demonstrated improved efficacy and good safety profile compared to approved drugs in type 2 diabetes patients in China. The long half-life of FCN-005 in human indicates it has a good potential to become a long-acting DPP-4 inhibitor to treat type 2 diabetes.
Currently, Phase 2 clinical trials of FCN-005 in type II diabetes patients are advancing in China. Fochon plans to leverage the clinical proof-of-concept data of FCN-005 from China to identify global partners for clinical development and commercialization.
Type II Diabetes and DPP-4
Type II diabetes is the most common form of diabetes, and accounts for 85-90% of diabetes cases diagnosed. High blood glucose in diabetes increases the risk for dangerous complications, including heart disease, blindness, and nerve and kidney damage. DPP-4 is the key enzyme responsible for degradation of intestinal glucoregulatory incretin hormones. However, expression of DPP4 is substantially dysregulated in diabetes. Inhibiting DPP-4 can potently lower blood glucose concentrations and reduce glycated haemoglobin (HbA1c) levels, which offers multiple clinically relevant advantages. Therefore, DPP-4 inhibitors, as a pharmacological class of blood glucose-lowering agents, open up new perspectives for the management of type 2 diabetes.