FCN-437
FCN-437 is a novel, proprietary and orally active inhibitor of Cyclin Dependent Kinase 4 and 6 (CDK4/6). It is being developed by Fochon as a monotherapy and in combination to treat solid tumor.
FCN-437 demonstrated much higher in vitro and in vivo potency and selective inhibitory activities against CDK4/6 compared to approved CDK4/6 inhibitors. FCN-437 exhibited broad anti-tumor activity in preclinical pharmacology models, favorable physical and pharmacokinetic properties, and improved toxicity profile in non-clinical studies. In particular, FCN-437 can distribute to the brain and provide an opportunity to treat tumors that have metastasized to the brain.
Fochon has received the approval from China Food and Drug Administration for the IND application of FCN-437 to treat patients with solid tumors in Jan 2018. Fochon announced today (June 25, 2019) dosing of the first patient in the US in the recently initiated Phase I clinical trials of FCN-437 in patients with advanced solid tumors.
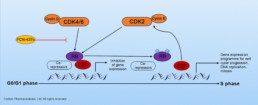
CDK4/6 can form complex with Cyclin D to phosphorylate Retinoblastoma protein (Rb), which enables cell cycle progression. Phosphorylation of Rb leads to dissociation of Rb from the E2F family of transcription factors and allows transcription of E2F target genes progression that drives cell cycle transition from G1 to the S phase. Loss of cell cycle control caused by aberrations in the CDK/Rb signaling pathway are common in a variety of solid tumors. Inhibiting CDK4/6 blocks CDK/Rb signaling pathway, which prevents cell cycle progression through the G1 restriction point, thus arresting tumor cell growth.